Nathan Sylte
Final Lab Report
Biology 319
The
Effects of Environmental Circadian Disruption on Mice
Introduction. Disruption
of circadian rhythms in humans is a common occurrence in modern life and is
believed to have serious health implications with regards to certain
pathological disorders, coronary heart disease, peptic ulcer disease, and
detrimental pregnancies (Knutsson 2003). However, it is not completely
understood what some of the specific consequences ECD (Environmental Circadian
Disruption) might have on growing animals and animals in general. This is
especially true with regards to how ECD might alter an animal’s daily activity
level. (Rice et al., 2008) showed chronic stress, specifically during the early
phases of the lives of mice, had long term consequences on their health. This
study specifically showed mice that had undergone chronic stress early on in
their lives exhibited disruption to their hypothalamic/ pituitary-adrenal
system. Another potential consequence of prolonged ECD would include increases
in mortality. Research has shown that the shifting and shortening of light
cycles causes an increase in mortality in mice (Davidson et al. 2006) (Park et
al. 2011).
So what can be concluded about our current understanding
of the effects of ECD and stress on animals? Stress during puberty has been
shown to decrease metabolic activity in male mice (Bastida et al. 2014). This
is important because physical activity is connected to metabolic activity
(Speakman and Selman 2003). Furthermore, research has shown regular and
prolonged ECD can lead to sleep loss in mice (Brager et al. 2013). This same
study also showed that chronic exposure to ECD led to an elevated inflammatory
response. These findings agree with (Majde and Krueger 2005) that showed sleep
disruption can be paired with alterations in the immune response. Furthermore,
stressful events have been shown to cause an increase in specific organ weights
in the spleen, heart, and adrenal glands in mice (Welch 1969). Stress can also
have affects on other organs as well. For example, prolonged stress can impact
the weights of thymus glands in mice, leading to a decrease in weight (Kubera
et al. 1998). Our interest is to examine the potential stress related outcomes
that ECD can have on mice. Specifically, we are interested in assessing the
possible affects regular daily rhythms have on growing animals with regards to
stress/anxiety levels, daily activity levels, and health.
Methods. A
total of forty-eight C57BL/6J mice between 4 to 5 weeks old were used. Mice were
housed in polypropylene bucket cages (22 x 22 x 44 cm) with wheels that
monitored activity levels. The cages allowed for easy access to food (Harlan
8604 chow) as well as water. Mice where divided into four groups which included
constant cycle females (n=12), constant cycle males (n=12), shifting females
(n=12), and shifting males (n=12). Mice treated with the constant cycle served
as controls. The experiment began with a total of twelve mice in each group,
with the animals being weighed before the experiment began, and then at 2-week
intervals after. Mice treated with the constant cycle were put on a twelve hour
light/dark cycle with the light being on from 6 am to 6 pm. The twelve hour
light cycle was not altered at any point during the experiment. Mice on the
shifting light cycle (mice undergoing ECD) were put on a twelve hour light/dark
cycle. However, the light/dark times were shifted eight hours earlier after
four days and eight hours later for the three remaining days. This shifting
pattern was kept for the entire length of the experiment.
After eleven weeks of living under their specific
light/dark cycles, mice were put through a test that tested their stress and
anxiety levels. The test involved placing mice in an elevated plus maze for
five minutes and was done within four hours of the midpoint of the light phase.
The elevated plus maze procedure used was based off of (Walf and Frye, 2007).
The elevated plus maze is a cross-shaped platform that is elevated 40 cm from
the floor. The arms of the plus maze are 30 cm long and 5 cm wide, with walls
surrounding two of them. The arms that are surrounded by walls are directly
across from each other, and only at the center are the walls absent to connect
the two sections. The procedure involved each mouse being removed from its home
cage, and then placed in the plus maze which is in a different room. The mouse
is placed in the center of the plus mazing facing the open arm. The handler
then moves out of the room, while the timer and recorder complete the test. The
number of entries into the open arms, number of entries into closed arms, time
in open arms, and time in closed arms are measured. After the five-minute test,
the handler took the mouse back to its home cage, and the interior of the maze
was cleaned with 70 percent ethanol to get rid of any odor the mouse may have
left. Mice that froze or jumped off of the maze were excluded from the plus
maze test.
After mice underwent the plus maze test, they were
euthanized humanely with carbon dioxide and specific organs were harvested,
then weighed. Organs were harvested in the following order and weights were
recorded: spleen, liver, adrenal gland, kidney, thymus, heart, and testis. Mice
that underwent altered light cycling were compared with controls. Organ weight,
body weight, elevated plus maze results, and daily activity levels of control
mice were compared. Data from two anapthalmic (lacking eyes) mice were excluded.
Unpaired T-tests and anova tests were used to analyze results, and Graph Pad
Prism 5.0 was used to create all figures. A Mann-Whitney test was used to
analyze entry results from the elevated plus maze test.
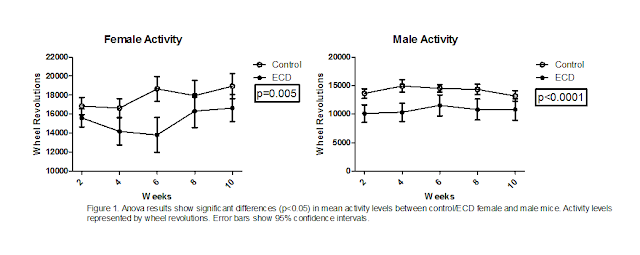
Results.
Activity levels between control and ECD mice were found to be significantly
different (fig 1). This was true for both female and male mice. Mice that had
undergone environmental circadian disruption displayed significantly less
activity levels. Male ECD mice had lower activity levels compared to male
control mice (p<0.0001), than female ECD mice did when compared with female
control mice (p=0.005). The differences found in activity levels between
control and ECD mice were the only significant differences found in the
experiment.
Although analysis of the data did not show any
significant differences (P>0.05) in organ and body weights, trends in the
data were found. When comparing adrenal gland weights between control and ECD
mice, the adrenal glands of ECD mice tended to weigh less than that of control
mice (fig 3). This was true for both female and male mice. However, the
differences in adrenal gland weights between control and ECD mice were not
significant in both female and male mice. While the adrenal gland weights of
ECD mice tended to decrease, spleen and thymus weights of ECD mice showed a
trend of increase (fig 3). The spleens of male ECD mice more greatly increased
in weight than the spleens of female ECD mice when compared with control mice.
Although spleen and thymus weights in both female and male ECD mice were
greater than that of control mice, the differences were not significant. Testis
weight in ECD mice also tended to increase when compared with testis weight of
control mice (fig 6). However, this difference in weight was proven to not be
significant. Male ECD mice showed a slight decrease in their liver and heart
weights compared to male control mice (fig 4). This trend was contrary to what
occurred in female mice. Female ECD mice presented liver and heart weights that
were slightly higher than that of female control mice (fig 4). In spite of the
fact there were differences between control and ECD liver and heart weights in
both female and male mice, these differences were not significant. There was
little difference shown when comparing female and male ECD mice kidney weights
with control mice kidney weights (fig 4). The slight increase in ECD mice
kidney weight that occurred in both female and males was not significantly
different than the kidney weight of control female and male mice. Final
differences in body weights between both female and male ECD mice also proved
to not be significant when compared with the final body weights of control mice
(fig 2). However, it should be noted that female and male ECD mice weighed
slightly less at the end of the experiment than control mice.
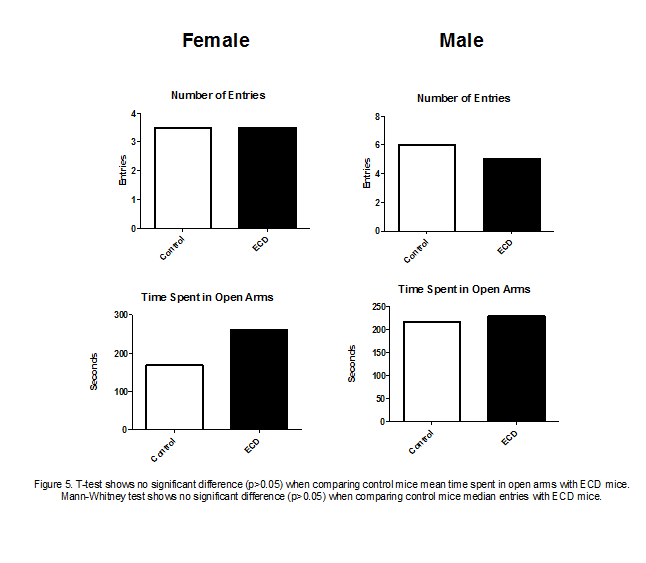
The elevated plus maze test results also yielded no
significant differences between ECD mice and control mice (fig 5). When
comparing the number of open arm entries of female ECD mice with female control
mice, it can be observed there was no trend in the data. However, male ECD mice
made slightly less open arm entries than male control mice. Analysis of the
amount of time mice spent in the open arms of the maze showed that both female
and male ECD mice generally spent more time in the open arms than control mice
did.
Discussion. Our
hypothesis that environmental circadian disruption would have affects on the
stress/anxiety levels, activity levels, and health of mice was partially
supported. This support comes from the activity data we collected (fig 1).
Research by (Speakman and Selman 2003) shows there is a relationship between
physical activity and metabolic rates. Furthermore, research has shown that
stress can decrease metabolic activity (Bastida et al. 2014). Therefore, it can
be thought that stress can have an influence on an animal’s daily activity
levels. Our findings agree with (Bastida et al. 2014) in that stress can impact
metabolic activity. In our case, it was possible ECD acted as a stressor to the
mice, which in turn potentially impacted their metabolic activity, causing them
to display lower activity levels than control mice did. Although we did not
directly measure metabolic activity in our experiment, the activity data
indicates metabolic activity may have been affected by ECD.
The organ and body weight data did not support the part
of our hypothesis that environmental circadian disruption would have
significant impacts on specific organs in mice. However, there are certain
trends in the data that agree with previous research. An example of this would
include the final spleen weights of ECD female and male mice (fig 3). The trend
we found was ECD mice had slightly enlarged spleens, though not significant
(p>0.05). These results agree with (Welch 1969), which found stressful
events can lead to the enlarging of the spleen, adrenal glands, and hearts of
mice. Contrary to what was found in their study regarding the enlargement of
adrenal glands as a result of stress, our results showed a trend of decrease in
adrenal gland weight (fig 3). Similarly, final heart weights (fig 4) were not
supported by (Welch 1969). They found stress led to an increase in heart
weight, whereas we found no significant difference of increase or decrease in
heart weight. We suspected thymus weight in ECD mice would decrease based on
the study done by (Kubera et al. 1998), which showed mice undergoing stress
displayed a decrease in thymus weight. However, our results were not supported
by their research. Our results showed there was a trend of enlargement of the
thymuses of both female and male ECD mice (fig 3), though it was not
significantly different. Lastly, we expected to see a decrease in the body
weights of ECD mice. Research has shown chronic stress can lead to a reduction
in body weight in mice (Jeong et al. 2013). Our results were not supported by
(Jeong et al. 2013), for we found there was no significant difference in final
body weight (fig 2). There may have been a reason for the lack of decrease in
body weight in ECD mice. When comparing activity data (fig 1) with body weight
data (fig 2), it can be seen that ECD mice ran significantly less, however they
weighed similarly to control mice. Therefore, it is possible the lower activity
levels concealed what otherwise would have resulted in a reduction in body
weight.
Our elevated plus maze results, to our surprise, did not
show ECD mice were more stressed or anxious than control mice (fig 5). The
elevated plus maze is commonly used to assess anxiety related behavior in
rodents (Walf and Frye, 2007). If ECD was indeed causing stress and anxiety in
mice, then we would have expected to see ECD mice behave more anxiously than
control mice. However, we did have several mice freeze or jump off the maze
during the test. This is extremely uncommon (Walf and Frye, 2007), and we did
not expect this to occur as much as it did. It is possible there may have been
something about the environment or the materials used in constructing the plus
maze that caused the mice to behave in the manner they did. Also, it is
possible the strain of mice we used (C57BL/J6) may behave non-anxiously in
general.
So what can be concluded about the effects of ECD as a
stressor on mice, and why is ECD a relevant topic for research? Although we did
not conclusively show ECD extremely affects and stresses mice, we did show ECD
can alter activity levels in mice. This means ECD may alter metabolic activity
in mice. Future studies might look at how ECD directly affects metabolic
activity in mice. Furthermore, a larger sample size should be used in the
future. The reason is we found certain trends in the weight data. A larger
sample size could potentially show, for example, ECD causes enlarged spleens in
mice. Overall, the research on circadian rhythms and shift work is extremely
important. The demands and commonplaces of modern life involve the disruption
of circadian rhythms in humans. A better understanding of circadian disruption
induced stress could lead to an increase in knowledge about potential health
problems associated with ECD.
References
Bastida, C.C.,
Puga, F., Lima-Gonzolez, F., Jennings, K.J., Wommack, J.C., Delville, Y. et al.
(2014). Chronic Social
Stress in Puberty Alters Appetitive Male Sexual Behavior and Neural Metabolic
Activity. Hormones and Behavior. Vol.
66(2): 220-227.
Brager AJ, Ehlen
JC, Castanon-Cervantes O, Natarajan D, Delisser P, et al. (2013) Sleep Loss
and the Inflammatory Response in Mice
Under Chronic Environmental Circadian
Disruption. PLoS ONE 8(5): e63752.
doi:10.1371/journal.pone.0063752.
Davidson, J.A.,
Sellix, M.T., Daniel, J., Yamazak, S., Menaker, M., Block, G.D., et al. (2006).
Chronic Jet-Lag Increases Mortality
in Aged Mice. National Institute of
Health. 16(21) :
R914-R916.
Jeong, J.Y., Lee,
D.H., Kang, S.S., et al. (2013). Effects of Chronic Restraint Stress on Body
Weight, Food Intake, and Hypothalamic Gene
Expressions in Mice. Endocrinology and
Metabolism. (Seoul, Korea). Vol. 28(4): 288-296.
Knutsson, A. 2003.
Health Disorders of Shift Workers. Occupational
Medicine.
Vol.53: 103-108.
Kubera, M.,
Basta-Kaim, A., Holan, V., Simbertsev, A., Roman, A., Pigareva, N., Prokopieva,
E., Sham, J. et al. (1998). Effect of Mild
Chronic Stress, as a Model of Depression, on the Immunoreactivity of C57BL\6
Mice. International Journal of
Immunopharmacology. Vol. 20(12): 781-789.
Majde, J.A.,
Krueger, J.M. 2005. Links Between the Innate Immune System and Sleep. Journal
of Allergy and Clinical Immunology. Vol.
116(6): 1188-1198.
Morgan JL, Svenson
KL, Lake JP, Zhang W, Stearns TM, et al. (2014) Effects of Housing
Density in Five Inbred Strains of Mice.
PLoS ONE 9(3): e90012. doi:10.1371/journal.pone.0090012.
Park, N., Cheon,
S., Hoon Son, G., Cho, S., Kim, K., et al. (2012). Chronic Circadian
Disturbance by a Shortened Light-Dark
Cycle Increases Mortality. Neurobiology
of Aging. Vol. 33: 1122.e11- 1122.e22.
Rice, C.J.,
Sandman, C.A., Lenjava, M.R., Baram, T.A. et al. (2008). A Novel Mouse Model
for
Acute and Long Lasting Consequences of
Early Life Stress. Endocrinology. Vol.
149(10): 4892-4900.
Speakman, J.R.,
Selman, C. 2003. Physical Activity and Resting Metabolic Rate. Proceedings of
the Nutrition Society. Vol.62:
621-634.
Walf, A.A., Frye,
C.A. 2007. The Use of the Elevated Plus Maze as an Assay of Anxiety Related
Behavior
in Rodents. Nature. Vol. 2(2):
322-328.
Welch, B.L.,
Welch, A.S. 1969. Sustained Effects of Brief Daily Stress (Fighting) Upon Brain
and Adrenal Catecholamines and Adrenal,
Spleen, and Hearth Weights of Mice. University of Tennessee.



No comments:
Post a Comment